The Zika Virus has gripped the world with panic, even reaching pandemic levels as it has reached beyond its original African/Asian borders into South America. The disease, spread through infected mosquitoes or through sexual intercourse, is especially dangerous for pregnant women, as it has been linked to microcephaly, a serious birth defect that causes brain deformation.
Scientists at the University of Pennsylvania have developed a point-of-care device for detecting the Zika virus for under $2 a pop. It’s about the size of a can of coke and can be operated by just about anyone, and even outside the clinic. A patient simply spits into the receptacle and after a while a paper test strip changes its color to a shade of blue if a Zika virus is detected.
Currently, and because of the recentness of the epidemic, diagnostic tests for the Zika virus are relatively limited. Additionally, the only tests that have been approved are not very accessible and require sensitive lab equipment, so having easy-to-use and low-cost testing devices could be a game-changer in combatting the Zika virus pandemic.
The innovative research, which was led by Research Assistant Professor Changchun Liu and Professor Haim Bau of the Department of Mechanical Engineering and Applied Mechanics in Penn’s School of Engineering and Applied Science, was recently published in the journal Analytical Chemistry.
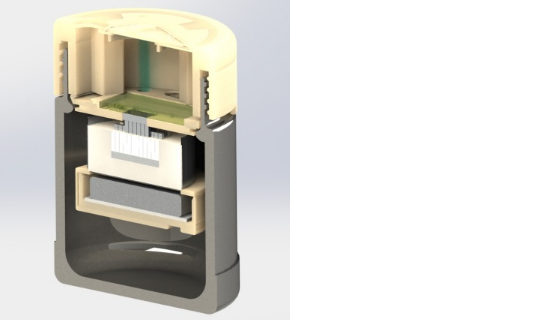
The new test uses a technique called loop-mediated isothermal amplification (LAMP) to grow the viral DNA. One problem of applying LAMP in the past has been developing so-called “primers,” short DNA strings that complement the DNA of the viral target. The researchers used computational techniques to identify spots on the Zika DNA that are unique to that virus, which were integrated into the detector. The other issue has been maintaining the proper temperature of the sample during the isolation and amplification of the DNA. To make that happen without electricity, the detector is situation within what is essentially an Thermos bottle.
Professor Haim Bau explains, “Although Zika primers for RT-PCR have been published in the literature, LAMP primers have not. So, using data mining, we identified highly conserved regions of the Zika virus genome that are divergent from other known pathogens. We then designed appropriate primers to recognize this sequence.”
Changchun Liu added, “In parallel, we engineered a low-cost, point-of-care system that consists of a diagnostic cassette and a processor. The cassette isolates, concentrates and purifies nucleic acids and carries out enzymatic amplification. The test results are indicated by the change in the color of a dye, which can be inspected visually.
From the study in journal Analytical Chemistry:
We report on a highly sensitive reverse-transcription loop-mediated, isothermal amplification (RT-LAMP) assay for rapid detection of ZIKV and its implementation in a simple, easy-to-use, inexpensive, point-of-care (POC) disposable cassette that carries out all the unit operations from sample introduction to detection. For thermal control of the cassette, we use a chemically heated cup without a need for electrical power. Amplification products are detected with leuco crystal violet (LCV) dye by eye without a need for instrumentation. We demonstrated the utility of our POC diagnostic system by detecting ZIKV in oral samples with sensitivity of 5 plaque-forming units (PFU) in less than 40 min. Our system is particularly suitable for resource-poor settings, where centralized laboratory facilities, funds, and trained personnel are in short supply, and for use in doctors’ offices, clinics, and at home.
For the device’s structure, the researchers also had to find a way to maintain a precise temperature for the samples without using electricity, which they achieved by creating a thermos casing with a self-contained heating element. For the top part of the device, the researchers 3D printed a specially designed lid capable of housing the genetic assay chip and the test’s other components. To use the test, the patient would simply have to put a sample of saliva into the device’s cartridge, and within about 40 minutes, color-changing paper reacted.
In the research study, the testing device was demonstrated using the researchers’ own saliva which had been injected with virus samples, and overall the results showed efficacy similar to the more complex RT-PCR tests. There is still more work to be done, however.
As Bau explains, “Our work represents a proof of concept at this stage. Before the assay can be adapted for medical use, we must experiment with patients’ samples and make assure that our assay and system match the performance of the gold standard and operate reproducibly and reliably. We are fortunate to have dedicated colleagues in endemic regions ready to assist us in this task.”
According to the researchers, the next step in the research process will involve demonstrating the 3D printed testing device’s selectivity, as well as developing a new test which could potentially quantify a viral load by using a fluorescent dye and an integrated smartphone camera.